It will be under the control of NHS Grampian.
Medicago, a biopharmaceutical company based in Canada, and GlaxoSmithKline (GSK) are launching the Phase 3 randomised, observer blinded, placebo-controlled study.
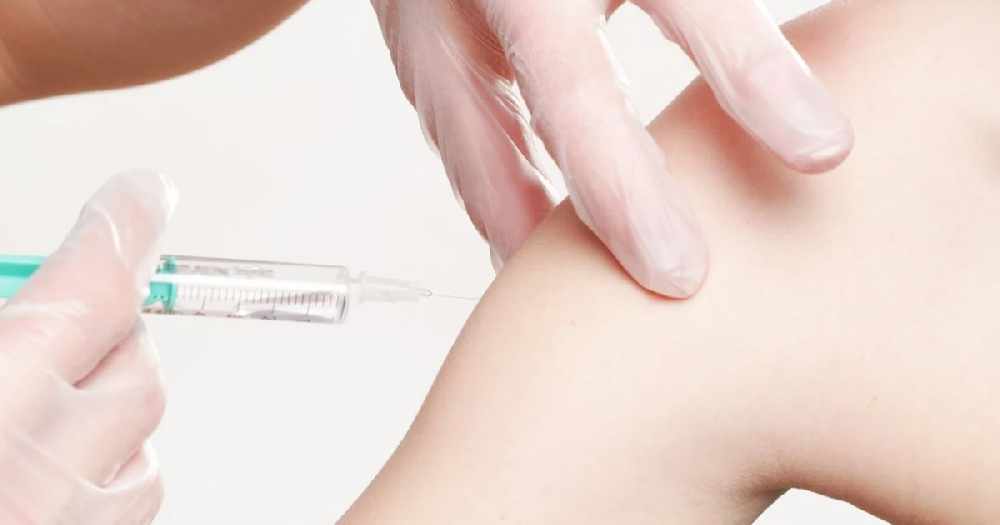
The study supported in the UK by the National Institute for Health Research (NIHR), NHS Research Scotland (NRS) and Health and Care Research Wales is the first to test a plant-derived COVID-19 vaccine candidate and will evaluate the efficacy and safety of the Coronavirus-Like Particle COVID-19 Vaccine (CoVLP).
1,500 volunteers will be recruited to the study within the UK, and each will receive an active study vaccine dose as part of the trial’s blinded crossover design.
Healthy adults between the age of 18 to 39-year-old will be asked to take part in the study, which will look to recruit over the course of the next four to six weeks.
Participants will receive two doses of the experimental vaccine and two doses of a placebo.
For both rounds of vaccinations, each two injections will be given 21 days apart.
Those who receive the CoVLP vaccine in Period 1 will receive the placebo in Period 2, while participants who receive the placebo in Period 1 will receive the experimental vaccine in Period 2.
Study participants will then be followed up for safety and immunogenicity for a period of 12 months after their last vaccination.
Several sites across England, Scotland and Wales, will run the Medicago vaccine study, in addition to multiple sites in the United States, Canada, Europe and Latin America.
Dr Roy Soiza, consultant physician at Aberdeen Royal Infirmary and study lead in Grampian said: “Clinical studies into COVID-19 vaccines remain critical to help find several safe and effective candidates to help protect us all. Volunteers in the Grampian region are still needed to help carry out these studies. We had an overwhelming response to previous vaccines trials and encourage interested participants to sign up to be contacted about taking part in COVID-19 vaccine studies.”


 Uber applied to launch in Aberdeen
Uber applied to launch in Aberdeen
 New scanners streamline security at Aberdeen Airport
New scanners streamline security at Aberdeen Airport
 Jimmy Thelin appointed new manager of Aberdeen Football Club
Jimmy Thelin appointed new manager of Aberdeen Football Club
 13°C
13°C
 12°C
12°C